|
| |
Home
:: Myths & Realities |

MYTH: Reusing plastic water bottles can cause them to break down into
carcinogenic compounds.
"Many are unaware of poisoning caused by re-using plastic
bottles. Some of you may be in the habit of using and re-using
your disposable mineral water bottles (eg. Evian, Aqua, Ice
Mountain, Vita, etc), keeping them in your car or at work.
Not a good idea. In a nutshell, the plastic (called polyethylene
terephthalate or PET) used in these bottles contains a potentially
carcinogenic element (something called diethylhydroxylamine
or DEHA). The bottles are safe for one-time use only; if you
must keep them longer, it should be or no more than a few days,
a week max, and keep them away from heat as well. Repeated
washing and rinsing can cause the plastic to break down and
the carcinogens (cancer-causing chemical agents) can leach
into the water that YOU are drinking. Better to invest in water
bottles that are really meant for multiple uses. This is not
something we should be scrimping on. Those of you with family
- to please advise them, especially children."
Reality: False.
This bit of plastic bottle scare lore is based upon a master's thesis from a University of Idaho graduate student, one which was unfortunately reported upon by the media despite its lack of peer review. According to The International
Bottled Water Association (IBWA):
The US Food and Drug Administration (FDA) regulates bottled water as a
packaged food product and, for bottled water and all other foods and their packaging, FDA has determined that PET meets standards for food contact materials.
The basis for [the e-mail was] a college student's masters thesis that was
not subject to peer review and did not reflect a level of scientific rigor that
would provide accurate and reliable information about the safety of these
products. Fortunately, FDA requires a much higher standard to make
decisions about food contact packaging. DEHA, as mentioned in the email is neither regulated nor classified as a human carcinogen. Further, DEHA is not inherent in PET plastic as raw material, byproduct or decomposition
product. DEHA has been cleared by FDA for food contact applications and
would not pose a health risk even if present. DEHA is a common plasticizer
used in many plastic items, many of which are found in the lab setting. For
this reason, the student's detection (see comment above) is likely to have
been the result of inadvertent lab contamination.
Also note that PET plastics used for bottled water containers are not unique
to this product type and is the same as PET plastics used to package other
common foods and beverages. 
(No “diethylhexyl adipate” (DEHA) is used in PET manufacturing. For details pl. refer Pl. refer FAQ of this note.)
The Environmental Protection Agency (EPA) at one time included DEHA on the list of toxic
chemicals maintained under the federal Emergency Planning and Community Right-to-Know Act
(EPCRA), but they have since removed it from the list because DEHA "cannot reasonably be
anticipated to cause cancer, teratogenic effects, immunotoxicity, neurotoxicity, gene mutations,
liver, kidney, reproductive, or developmental toxicity or other serious or irreversible chronic
health effects." And, according to the International Agency for Research on Cancer (IARC),
diethylhexyl adipate "is not classifiable as to its carcinogenicity to humans."
Some organizations (including the IBWA) do recommend that plastic water bottles be used only
once before recycling, but not because re-use is likely to cause carcinogenic compounds to leach
from the plastic bottles into the liquids they hold. The concern is that people (particularly
children) can too easily spread and ingest bacteria from their hands and mouths by re-using bottles
without properly washing them or allowing them sufficient time to dry.
FREQUENTLY ASKED QUESTIONS (FAQ) on PET
Will a plastic bottle leach harmful substances into water if I reuse it?
Most convenience-size beverage bottles sold in the U.S. are made from
polyethylene terephthalate (PET). The FDA has determined that PET meets
standards for food-contact materials established by federal regulations and
therefore permits the use of PET in food and beverage packaging for both single
use and repeated use. FDA has evaluated test data that simulate long-term storage
and that support repeated use.
The toxicological properties of PET and any compounds that might migrate under
test conditions have also been well studied. The results of these tests demonstrate
that PET is safe for its intended uses. (For details, see The Safety of Polyethylene
Terephthalate.)
What about the student project that claimed to have found unhealthy
compounds in water samples from reused bottles?
What about the student project that claimed to have found unhealthy
compounds in water samples from reused bottles?
The subject of a widely circulated e-mail hoax, these claims stem from a
University of Idaho student’s masters thesis that was promoted in the media but
was not subject to peer review, FDA review or published in a scientific or technical
journal.
While the student project may have been suitable work for a masters thesis, it did
not reflect a level of scientific rigor that would provide accurate and reliable
information about the safety of these products. Fortunately for consumers, FDA
requires a much higher standard to make decisions about the safety of food contact
packaging.
But I read that the student’s project found carcinogens?
The student’s thesis incorrectly identifies di(2-ethylhexyl) adipate (DEHA), a
plastics additive, as a human carcinogen. DEHA is neither regulated nor classified
as a human carcinogen by the U.S. Occupational Safety & Health Administration,
the National Toxicology Program or the International Agency for Research on
Cancer, the leading authorities on carcinogenic substances.
In 1991, on the basis of very limited data, the U.S. Environmental Protection
Agency classified DEHA as a "possible human carcinogen." However, in 1995, EPA
again evaluated the science and concluded that "...overall, the evidence is too
limited to establish that DEHA is likely to cause cancer."
Further, DEHA is not inherent in PET as a raw material, byproduct or
decomposition product. DEHA is a common plasticizer that is used in innumerable
plastic items, many of which are found in the laboratory. For this reason, the
student’s detection of DEHA is likely to have been the result of inadvertent lab
contamination. This is supported by the fact that DEHA was detected infrequently (approximately 6% of the samples) and randomly, meaning that the frequency of
detection bore no relationship to the test conditions.
Moreover, DEHA has been cleared by FDA for food-contact applications and would
not pose a health risk even if it were present.
Finally, in June 2003, the Swiss Federal Laboratories for Materials Testing and
Research conducted a scientific study of migration in new and reused plastic water
bottles from three countries. The Swiss study did not find DEHA at concentrations
significantly above the background levels detected in distilled water, indicating
DEHA was unlikely to have migrated from the bottles. The study concluded that
the levels of DEHA were distinctly below the World Health Organization guidelines
for safe drinking water.
Is it true that the U.S. Food and Drug Administration (FDA) only allows plastic
beverage bottles, such as those made with polyethylene terephthalate (PET),
for one-time use?
No, FDA allows PET to be used in food-contact applications, including food and
beverage packaging, regardless of whether the packaging is intended for single or
repeated use. PET beverage bottles sold in the United States are designed for
single use for economic and cultural reasons, not because of any safety concerns
with PET.
In fact, refillable bottles made with the same PET resin as single-use bottles are
safely reused in a number of other countries. The only difference is that refillable
bottles have thicker sidewalls to enable them to withstand the mechanical forces
involved with industrial collection and commercial cleaning and refilling
operations.
Can freezing a PET beverage bottle cause dioxins to leach into its contents?
This is the subject of another e-mail hoax. There simply is no scientific basis to
support the claim that PET bottles will release dioxin when frozen. Dioxins are a
family of chemical compounds that are produced by combustion at extremely high
temperatures. They can only be formed at temperatures well above 700 degrees
Fahrenheit; they cannot be formed at room temperature or in freezing
temperatures. Moreover, there is no reasonable scientific basis for expecting
dioxins to be present in plastic food or beverage containers in the first place.
Compiled by :
PET Marketing Technical Services

Phone: 022 –3032 22012 / 2251 / 2252
Source: http://www.plasticsinfo.org/beveragebottles/faq.html
|
 |
POINT: Plastics / plastic
bags are harmful to plants & the soil
Counter Point:
- Plastics protect plant life in multiple ways
- Plastics prevent massive deforestation by offering wood
substitutes. eg. Furniture, building materials, crates
- Plastic pipes are used extensively in Irrigation &
Water Management
- Flood Irrigation, Sprinkler Irrigation, Micro Irrigation
(Drip/Trickle) etc
China uses One million tonnes of PE in agricultural application.
POINT: Plastics are not recyclable
Counter Point: Plastics are
100% recyclable via various routes :
- Mechanical recycling : Plastics can be recycled several
times into economically useful low cost products eg. Footwear,
mats, sewer pipes etc.,
- Waste plastics are also recycled without sorting into
synthetic lumber / wood products like rails, fencings, posts,
benches and land scaping products.
- Plastics can be thermally recycled / incinerated to recover
energy
- Plastics can be chemically recycled to recover monomer
for reuse.
In India we already recycle 60% of plastics from both Industry
and urban waste streams Vs world average of 20-25%.
POINT: Plastics deplete precious
& scarce fossil fuel
Counter Point: The different
uses of commercially produced oil.
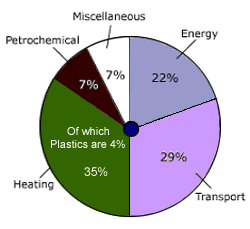
Plastics use globally only 4% of commercially produced oil.
The rest being accounted by transport, energy and others.
Infact plastics add value and extend life of fossil fuel instead
of burning it directly.
POINT: Plastics are toxic
and are not safe for usage
Counter Point: Plastics are
used worldover safely for personal care products, packaging
of food & medicine, in-vitro medical applications and
for child care products.
- Toothbrush, toothpaste tubes, shampoo bottle
- Milk pouch, edible oil container, ice cream pack
- Blister packing - tablets and capsules
- Medical disposables - IV bags, blood bags, gloves
- Heart valve, hip joint
- Toys, diapers
Food and drugs authorities worldwide permit use of different
plastics in various applications. Industry needs to adhere
to prescribed standards.
POINT: Plastic bags contain
plasticizers
Counter Point:
- Plastic bags are made from Polyethylene (PE) which is
a polymer of pure Carbon & Hydrogen. The material by
itself is soft in nature. No plasticizers are used / required
for any Polyethylene application including Poly Bags.
- The campaign that Plastic bags contain plasticizers is
a malicious canard
- Plasticizers are used only in PVC Products.
POINT: Plastic bags contain
titanium dioxide and lead based components which are toxic
& Dyes used in coloured bags cause severe health hazards.
Counter Point:
- Most of the pigments used for making bags are organic
in nature. Use of lead or cadmium based compounds does not
arise at all.
- The inorganic pigments used in plastics do not contain
lead or cadmium.
- Organic pigments which are used are compatable with the
polymer to get bonded. They cannot leach out.
Industry has accepted to use natural unpigmented carry bags
for food contact applications. Recycled bags will be coloured
(using BIS approved pigments) for other applications.
Next »
|